Introduction: Despite the variable course of MCL, limited prospective markers exist to identify more indolent disease. MCL is often diagnosed in older patients (pts) less able to tolerate intensive approaches. Bendamustine/rituximab (BR) is a standard non-intensive regimen for MCL, and use of MT remains controversial. Emerging data in MCL suggest a correlation between achieving MRD negativity (MRD-) and progression-free survival (PFS). The GALLIUM study (Marcus R, et al, N Engl J Med 2017) previously showed improved PFS with a bendamustine/obinutuzumab (BO) induction compared with BR in follicular lymphoma, and we postulated this benefit may be observed in MCL. Using MRD testing, we predicted that MT may be omitted in pts achieving MRD- status after induction BO chemoimmunotherapy for MCL.
Methods: Adult pts ≥18 years (yrs) with previously untreated MCL who were ineligible for more intensive therapies (including transplant) were enrolled. Pts received 4-6 CY of BO induction intravenously (IV) every 28 days (D) with B 90 mg/m2 IV D1 & 2 every 28D for 6 CY concurrent with O IV (cycle 1 = 100 mg D1, 900 mg D2, and 1000 mg D8 & 15; then 1000 mg D1, CY 2-6). Peripheral blood (PB) MRD was obtained using NGS (ClonoSEQ®) after cycle 2 BO as an exploratory endpoint. Consolidation O (CO) 1000 mg IV weekly for 4 doses was initiated ≤12 weeks after the final dose of BO. Restaging and MRD testing of PB and bone marrow aspirate (BMA) was done ≤30 days after CO completion. For pts with complete response (CR) radiographically and MRD- in PB/BMA, maintenance O (MO) was omitted. For pts without CR or persistent MRD positivity (MRD+) in the PB or BMA, MO was given with 1000 mg IV D1 of a 56-day cycle for 8 CY. Pts were followed for ≥2 years from therapy completion. The primary endpoint is PFS from D1 of BO induction. Secondary endpoints are MRD status, concordance rate of PB/BMA in predicting MRD status, response rates and overall survival (OS).
Results: Twenty-one pts (of planned 32) enrolled beginning 2/2018, with follow-up through 6/1/2023. The study closed prematurely due to slow enrollment. Median age is 70 yrs with 3 pts >80 yrs; majority were men (76%). Median MIPI score is 6.11 and median ECOG performance status 0. Twenty pts (95%) have stage IV disease.
Twenty pts completed 4 (n=1) or 6 CY BO. Ten pts received MO; 10 did not receive MO per protocol due to radiographic CR and MRD- PB/BMA post-CO. Six pts did not complete MO for reasons of progressive disease (n=4), infection (n=1) and new carcinoma (n=1). After cycle 2 BO, PB analysis was MRD+ in 7 pts and MRD- in 13. Concordance rates between post-CO MRD testing in PB and BMA was 70% (14/20). Of non-concordant values, all had PB MRD- but BMA MRD+.
Best responses in 20 assessable pts are: CR 75% (15/20), partial response 20% (4/20), and stable disease 5% (1/20).
Most common grade 3/4 toxicities were: neutropenia (10/21), leukopenia (6/21), infections (5/21) and infusion reactions (2/21). One pt had grade 5 cardiac arrest with sepsis during BO. Most common grade 3 infections were abdominal/colitis (n=3) and pneumonia (n=2). Most common grade 1/2 toxicities were nausea (14/21), diarrhea (10/21), constipation (8/21), dysgeusia (6/21), fatigue (8/21) and dry skin/rash (12/21).
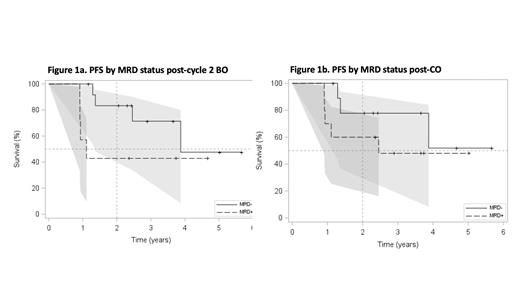
After a median duration of follow-up of 43.9 months (mos) (95% confidence interval [CI] 28.3-60.1), PFS at 2-yrs and 3-yrs is 66.0% (95% CI 41.6-82.2) and 58.7% (95% CI 33.2-77.3), respectively; and median PFS is 46.5 mos (95% CI 16.4-∞). MRD- status after the exploratory endpoint post-C2 BO was predictive of 2-year PFS (Figure 1a), 83.3% (95% CI 48.2-95.6) for MRD- vs. 42.9% (95% CI 9.78-73.4) for MRD+, with hazard ratio [HR] 0.34 (95% CI 0.09-1.39), p value 0.133. Evaluation of PFS by MRD status post-CO showed similar outcomes regardless of receiving MO (Figure 1b): 2-year PFS is 77.8% (95% CI 36.5-93.9) in MRD- pts post-CO vs 60.0% (95% CI 25.3-82.7) in MRD+ pts; HR 0.45 (95% CI 0.10-1.91, p value 0.278. Median OS is 46.5 mos (95% CI 35.1-∞).
Conclusion: Omission of MO in pts achieving MRD- status after induction/CO did not result in worsening PFS. Observed PFS and toxicities with BO induction and CO appears comparable with other data sets utilizing a BR-based induction +/- MT in older, less fit MCL pts. Correlation between PFS and PB MRD testing after C2 induction therapy represents a potential new prognostic marker of MCL behavior, and may be an important early disease indicator in development of risk-adapted therapies.
OffLabel Disclosure:
Kenkre:Epizyme: Research Funding. Pophali:SeaGen: Honoraria. Mattison:Nkarta: Membership on an entity's Board of Directors or advisory committees. Chang:Genentech: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; MorphoSys AG: Consultancy; Adaptive Biotechnologies: Research Funding; Genetech: Research Funding.
Obinutuzumab use will be described for induction and maintenance therapy for mantle cell lymphoma

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal